1. Abstract
I postulate that the stage for Multiple Sclerosis (MS) onset and ongoing disease progression is set by chronic deficiencies of vitamin D3 and/or Vitamin K2 as menaquinone 4 (MK4). I will describe my MS history and improvement from Relapsing Remitting MS to Non Progressive Non Remitting MS as evidenced by quieted symptoms and stabilization of white matter lesion load lasting 20 years, without medications. I will offer my approach to finding wellness, milestones along the way, finding what worked and ultimately, with emerging research, describe the how and why. The implication for other disorders and diseases is evident. I will primarily focus on MS.
2. Introduction
The inflammation response is critical to protect and maintain a healthy body. Just as critical is that this response shut off at the right moment. Matrix Metalloproteinases (MMP) serve as a gateway at the Blood Brain Barrier (BBB), allowing, then disallowing the inflammation response to pass. When the gateway strays open beyond the correct window, a cascade of negative activities ensue [1,2]. I postulate that Vitamins D3 and K2 as MK4, in partnership, serve to regulate this gateway and thus maintain a proper inflammatory response. Diet, and a properly balanced micro gut flora support vitamin K2 MKn . Vitamins D3 and K2 MK4 and MK7 can be supplemented. However, there are many opportunities for K2 assimilation to be blocked, leading to a deficiency, see section 8. To make it more difficult, there are no in clinic tests available for K2. I relied upon trial and response and periodic MRI exams.
3. My Experience and Milestones
In 1995 I was first studied and had three white matter lesions. I thought I dodged a bullet. Then, in 2000, I was diagnosed with relapsing remitting MS and placed on Betaseron for a year, followed by Avonex for another 18 months. My case nurse urged me to address diet and lifestyle. I started addressing oxidative stress by loading up on antioxidants. An alkalizing diet and supportive supplements did help with symptoms. My major symptom was heat fatigue. This symptom emerged when my environmental temperature reached above 72 degrees F. Increased serum Nitric Oxide (NO) blooms relax blood vessels to regulate body temperature. This elevated NO brought on debilitating fatigue. In 2003, I successfully controlled this symptom with Inosine supplementation. Inosine increases adenosine triphosphate (ATP) which degrades to uric acid which in turn scavenges NO. It was effective within minutes but there was a side effect. I developed painful uric acid crystals in my big toe, gout. Recent research expanded understanding of inosine’s role [3,4,5]. The key here is NO flux. Lowering it becomes an important clue later. Meanwhile, my lesion load continued to increase.
I began to focus on soft tissue calcification. In 2005 I experimented with a calcium detox regimen that involved tetracycline and ethylenediaminetetraacetic acid (EDTA). I discontinued MS meds and continued this for three and a half years. If I paused this regimen for a few days the benefit retracted. This is when my lesion load stabilized as evidenced by a 2005 MRI baseline that has held since. The key here is calcium carbonate, see 5v.
Long term tetracycline imposed a toll so I looked to how my body naturally decalcifies soft tissues. In 2008 soft tissue calcification was still my interest. Vitamin K2 was emerging in the literature for it’s decalcifying role. Of the two menaquinone available at the time, MK4 and MK7, I supplemented each separately for response. MK4 was effective in quieting my MS while MK7 was not. K2 MK4, at that time, was not well understood. It was what clearly worked for me. My vitamin D3 measured 20 ng/mL. Supplementation ensued and the D3/K2 MK4 partnership came together. Currently the role of MK4 is better understood, see 4, 5ii, iii, iv. My symptoms remained quiet and my lesion load remained stable.
Over time I sustained wellness and in 2015 requested a second opinion of my MS diagnosis. My diagnosis was changed from relapsing, remitting to non progressive, non relapsing. I’ll take it.
In more recent years understanding of the various K2 MKs evolved. MK4 indeed has focus on the brain and the immune response. MK7 has focus of calcium management, directing calcium to bone and teeth and not to soft tissues. My interest shifted from calcification to the effect upon MMP at the BBB which MK4 serves a critical role [6]. The differences and roles are discussed in section 5.
In 2022 I had an emergency cholecystectomy, I lost my gall bladder. This brought my attention closer to the role of bile salts in vitamin K2 assimilation, LDL cholesterol as transporter, and all they ways K2 assimilation can be disrupted [7,9]. See section 8.
Most lately, as is common in a 70 year old male like me, I developed an enlarged prostate. An alpha blocker, Tamsulosin, was prescribed. It works as intended but clouds my MS regimen. This alpha blocker can raise NO. More alarming is that it acts to block dopamine receptor D2 [9,10,11,12]. I am currently wrestling with this question.
Here’s what I have learned. Supporting Science, Current Research and the Hypothesis.
4. The Role of the Vitamin D3/K2 MK4 Team
When proper levels of Vitamins D3 and K2 MK4 are maintained they work as a team to regulate, as examples, IL-6, IL1B, TNFa, and C Reactive Protein levels thus keeping them from rising inordinately high when activated. D3 and K2 MK4 in turn support, as examples, GAS 6, and Protein S levels when activated. By doing so, the inflammation response is right sized. Inflammation levels instruct inducible nitric oxide synthase (iNOS) levels in a direct relationship. When inflammation increases, so does iNOS and alternately when inflammatory response decreases so does iNOS, one to one. Inflammation translates into NO elevation
[13,14,15,16,17,18,19,20,21,60].
Elevated iNOS induced NO deactivates MMP at the BBB. Deactivated MMP at the BBB means that the inflammation response gateway is open. The mechanism is inhibition of protease activity due to interaction with or release of Zinc2+ in cystine switches, the protein unfolds. Higher NO translates into continued inflammation response while low NO means a timely shutting off of inflammatory response [1,2,5,14,18,19,20,21,22,23,24,25].
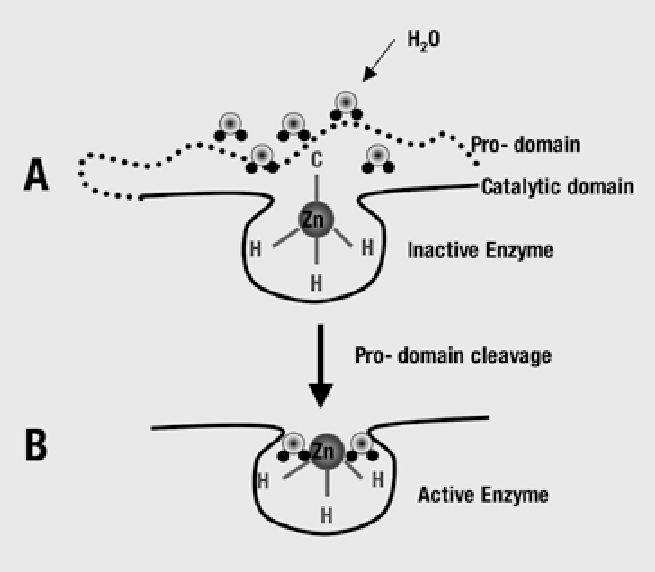
A vitamin D3 and/or K2 MK4 deficiency leads to a failure to hold inflammatory response amplitude within range and allows inordinate NO levels to persist.
5. The Core Supplements
i. Vitamin D3
Multiple Sclerosis has an interesting epidemiological clue. MS propensity exists at higher and lower latitudes. MS is rare for those raised on the equator. Logically vitamin D absorption from sunlight varies seasonally as the higher latitudes are seasonally tilted toward and away from the sun. Living in Scotland or Tasmania means one must build up stores of vitamin D acquired in the summer for winter needs when sunlight is diminished. This leaves a risk of being low in D3. Vitamin D3 is low in many populations in these regions. The good news is that it can be successfully supplemented. It is recognized that D3 should be supplemented in MS patients, commonly 5000IU daily [26]. I do.
ii. Vitamin K2
Vitamin K2 is like the B vitamins in that there are an array of K2 versions each with a different role. It is a fat soluble vitamin along with A, D and E. Unlike the other three, K2 has a very short half life in our systems and is not stored, less that a day to several days depending on which MK. We need to replenish it daily. Another difference is that it’s transporter is LDL cholesterol, the “bad cholesterol”. See section 6. K2 has a isoprenoid side chain, menaquinone (MK). The length of this chain defines the MK designation. MK4 is the shorter. Research is underway to understand these [7,9,13,17,27,28,29,30,31,33,34,35].
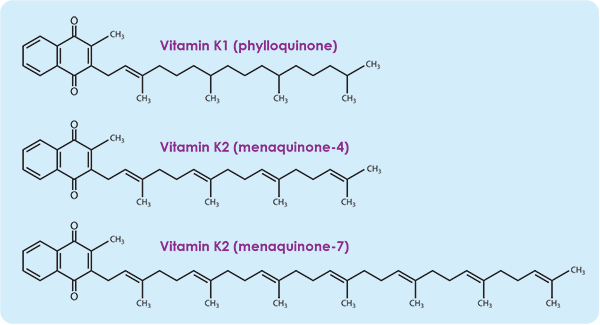
iii, Vitamin K2 MK4
MK4 has a shorter chain and differs significantly from the longer chain MKs. As omnivores, we acquire K2 MK4 from our diets. We get vitamin K1 from leafy greens, some of which is inefficiently synthesized into K2 MK4. Some K2 MK7 will will shorten into MK4. See section 4iv. Mostly we acquire MK4 from eating animal proteins sourced from herbivores, animals that eat grass, produce K1, synthesize K1 into K2 MK4 and store it mostly in their organs. Chicken/duck/goose liver pate, egg yolk, chicken dark meat, beef and lamb liver, pasture source butter are examples of sources. Curiously, these are foods that we are told to avoid? MK4 is by far the most prevalent K2 in our brains and bodies. Again, the good news is that we can supplement it. I take 5mg daily. Natural forms of K2 have the correct isoprenoid. The bad news is that the synthesis of K2s can be disrupted and this is likely the reason we are widely deficient [28]. See section 8.
iv. Vitamin K2 MK7
The MK7 version of K2, like all MKs longer than MK4, are derived from bacteria in fermented foods. Natto is the greatest source of MK7, with sauerkraut, kefir/yogurt, hard cheeses, and Edam cheese, all not pasteurized, as other examples. MK7 is the most studied so far. Its role is to manage calcium. It does this by carboxylating MGP. A carboxylated MGP holds onto calcium while transporting it to bone and teeth. Un-carboxylated MGP does not retain calcium and allows the calcium its carrying to precipitate into soft tissues while in transit. Diet plays a role with the bacteria based MKs. What we consume feeds the bacteria in our gut. Bacterial gut species thrive on a diet they prefer and conversely don’t thrive on a diet not suited to them. So feed the good bacteria and starve the bad. This is a growing field of study and promises to lead to a powerful understanding of the role of our gut flora and the rest of our body’s wellbeing. MK7 has a longer half life than MK4 and some will step down to MK4. They have different roles, so both need support, MK4 for a stable immune response like in MS and MK7 for heart, circulation and bone health. I take 100 mcg of K2 MK7 daily. 100 to 300 mcg daily is mentioned in the literature [35,36,37].
v. Calcium Citrate
Calcium is important. The right calcium is critical. Previously, I had supplemented calcium carbonate. As mentioned before, it was when I performed and continued to perform a calcium carbonate detox that my lesion load stabilized and symptoms remained quiet. See section 8. I stopped supplementing calcium carbonate and now supplement calcium citrate instead. I take 600mg daily of calcium citrate. It’s really hard to avoid calcium carbonate, it’s in everything.
vi. Magnesium Glycinate
Magnesium and calcium are codependent. I take magnesium glycinate that pairs well with calcium citrate, 300mg daily. I can take an additional dose of magnesium if and when I develop a muscle cramp for quick relief.
vii. Bile Salts
As I mentioned before, my gall badder was removed under emergency circumstances. Bile salts are critical in vitamin K2 assimilation with LDL cholesterol for transport to the “job site”. Having lost proper bile salts management, I must supplement bile salts. I use multiples of low dose, 125mg, so that I can match bile dosing with animal proteins in my meal, especially and together with my K2 supplements. I avoid the discomfort of a larger bile salt dose. Let me note here that we are far too casual about removing gall bladders. I had to, but be convinced that you must before you allow this procedure [7].
xiii. Other Supplements I Take
Lactobacillus acidophilus
Psyllium fiber
Vitamin B Complex
Zinc, I suggest you test for Zinc and Copper and supplement if needed and accordingly.
6. LDL Cholesterol
The “bad cholesterol”. It turns out that LDL is critical for K2 transport. Ever since I started supplementing K2 and D3, my LDL has drifted into healthy regions and has sustained a healthy ratio with HDL. Triglycerides are thought to be unaffected. Maintained long term correct levels of D3 and K2 in fact bring LDL into healthy range and maintain healthy cholesterol balance [8,9,38].
7. Suggested Lab Work
I receive a MRI every three years to check on my lesion status, its my report card. So far I have an A++. I suggest lab work for vitamins A, D and E, calcium, magnesium, fasting lipid levels and C reactive protein. I tested for oxidized LDL (OXLDL) in an effort to imply vitamin K2 levels. I was low (50), that’s good [40,41].
The K2 tests that exist are for research and test for affected proteins, ie carboxylation ratio of serum human MGP for testing K2 MK7 to imply K2 status.
8. Inhibition of Vitamin K2 Synthesis
There are many known inhibitors of K2 synthesis and some I suspect. Here is a partial list.
Calcium carbonate is commonly used as a phosphate binder to help lower levels in chronic kidney disease, hyperphosphatemia. It is known that its use can lead to vascular calcification. Turns out that calcium carbonate inhibits K2 synthesis. Calcium carbonate is used throughout our food chain, it is cheap. Read labels. We must lobby the food industry to remove calcium carbonate from our food supply. It is a challenge and probably what blocked my K2 synthesis and contributed to my MS [42,43,44,45].
Statins are known to inhibit vitamins D3, K2 and Q10. Atherosclerosis and heart disease are known risk factors. Again, statins inhibit K2 synthesis. This is why statins present a risk of increased coronary artery calcification. Long term stable vitamin D3 and K2 levels maintain healthy cholesterol levels and balance [8,37,43,46].
Chronic radon exposure is hypothesized to lead to MS. Radon has a 72 hour half-life, enters the body through the lungs and has an affinity with fat tissues. I suggest that chronic exposure to radon inhibits LDL synthesis with K2 [47].
Broad spectrum antibiotics disrupt gut flora balance and can lead to some vitamin K2 deficiencies [35,36,38].
A highly acidic diet leads to a poorly balanced gut flora and poor production of longer side chain MKs. A North American diet tends to be acid forming.
Bile acid medications lower bile salts and may interrupt vitamin K2 synthesis [43].
Orlistat prevents the absorption of dietary fats in the intestines and can disrupt vitamin K2 synthesis [43].
Epstein Barr virus’ relationship with cholesterol suggests a possible role in inhibiting vitamin K2 synthesis. Additionally, Epstein Barr virus may take advantage of a existing vitamin K2 deficiency having left the MMP gate open too long [38,49].
Cholecystectomy, loss of gall bladder, disrupts LDL cholesterol and vitamin K2 synthesis [7,9,10,11,12,50].
In 2005, Direct-MS posted my hypothesis that background ionizing radiation from cosmic radiation showers may have a role in MS. I noted that protons from our sun and from supernovae enter out planet’s magnetic field as charged particles where they are captured and guided to northern and southern latitudes. This distribution overlays MS distribution, high MS in Scotland and Tasmania, while low MS on the equator. I suggest that aggregate MS diagnosis peaks while tracking and slightly lagging the “eleven year” solar cycle when sunspot activity is peaking and proton flux is greatest. Melanin provides protection from ionizing radiation and deserves consideration as well.
Geography of Multiple Sclerosis
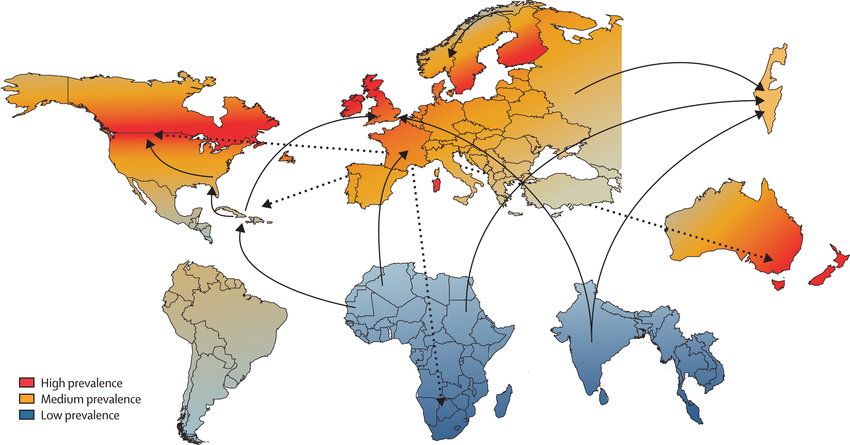
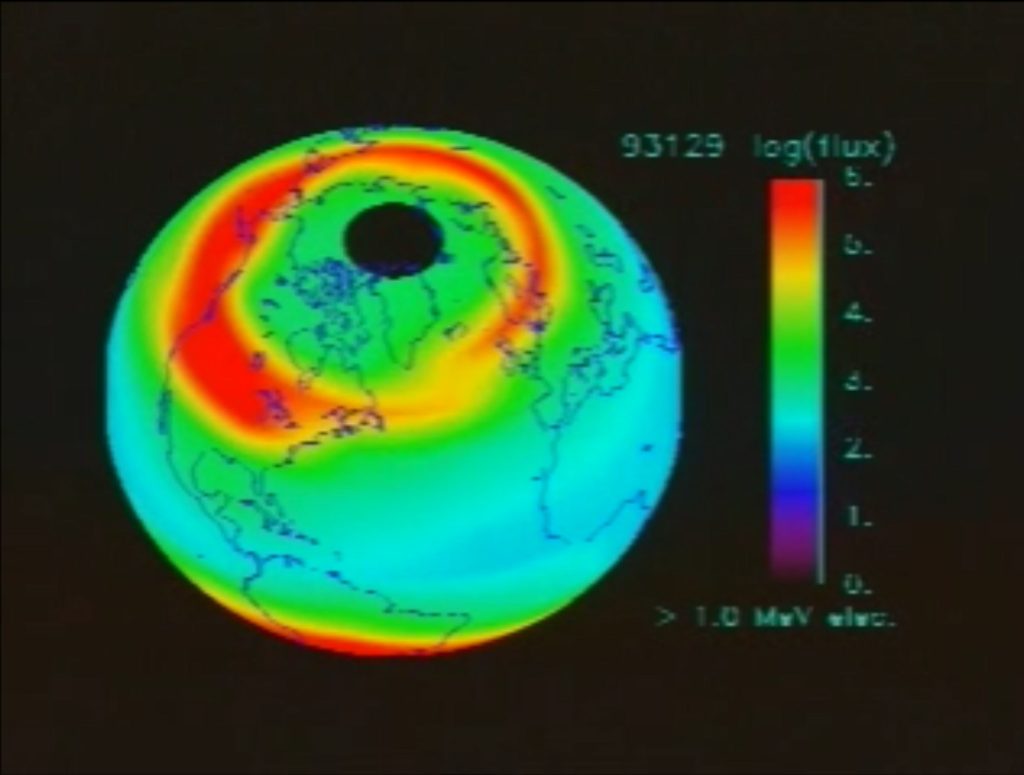
Source: [https://archive.org/details/SVS-89]
See: [https://www.spaceweather.gov/] for live proton flux data and images.
An informal study that my son and I conducted yielded an inverse relationship between barometric pressure and MS experienced symptom levels. As a filter, atmosphere density serves to absorb energy. High pressure absorbs more energy and low pressure less. High symptom expression is paired with low pressure. After 20 years of considering the connection I believe that proton progeny (sub particles), after passing through our atmosphere and then through us, damage tissues. A sterile inflammatory response ensues to address this damage and loads this inflammation and resultant NO on top of normal inflammation and NO levels [51]. Living in these latitudes presents potentially chronic higher levels of inflammation response. If we are low in D3 and/or K2 MK4, this added inflammation and NO load further delays closing of the MMP gateway at the BBB. Its “salt” in the K2 deficit wound.
9. My Core Vitamin Regimen
Vitamin D3, 5000IU daily
Vitamin K2 MK4, 5 mcg daily
Vitamin K2 MK7, 100 mcg daily
Calcium Citrate, 600 mg daily
Magnesium Glycinate, 300 mg daily
Vitamin B complex
Bile Salts Booster, multiples of 125mg as needed (because my gall bladder is missing)
10. Next Steps
The single most important tool we need now is an in clinic lab test for each vitamin K2 MK, especially MK4 and MK7 [52,53,54]. We must establish recommended healthy ranges. We need to include the effects upon vitamin K2 synthesis when vetting drugs so that cost benefit evaluations include effects upon vitamin K2. We need to support research. Most research is happening in Europe and Japan. Finally, we need to bring our medical practitioners along.
11. The Wrap
I believe that the theorized “causes” of MS, and many other disorders and diseases, are in fact causing a deficit of vitamin K2 or finding an existing K2 deficiency an opportunity. A deficit of K2 is the cause, the agents that block K2 synthesis are the triggers. Once we understand the triggers we a can work to remove them. Then with diet and supplementation we can achieve wellness. Closing the D3 and/or K2 deficiency door will keep unwanted “guests” out. This is my hypothesis. I have maintained wellness for twenty years while having an MS diagnosis on board for thirty. Naturally, a hypothesis will and should adapt to advances in research and better understanding, as will this one. Importantly, my MS stopped and remains quiet with continued supplementation of vitamins D3 and K2 as MK4, for 20 years.
Scott Farmer
November, 2025
Some COVID reading you’ll now appreciate [16,55,56,57,58,59].
References
1. The role of cholesterol metabolism in multiple sclerosis: From molecular pathophysiology to radiological and clinical disease activity. Balazs Lorincz, Elizabeth C. Jury b, Michal Vrablik c, Murali Ramanathan d, Tomas Uher a e, et al, 2022. [https://www.sciencedirect.com/science/article/abs/pii/S1568997222000581]
2. Matrix metalloproteinases (MMPs) family gene polymorphisms and the risk of multiple sclerosis: systematic review and meta-analysis. Mina Mohammadhosayni, Arezou Khosrojerdi, Keivan Lorian, Saeed Aslani, Danyal Imani, et al, 2020 .[https://link.springer.com/article/10.1186/s12883-020-01804-2]
3. Inosine – a multifunctional treatment for complications of neurologic injury, Claire Doyle, Vivian Cristofaro, Maryrose P Sullivan, Rosalyn M Adam, 2018. [https://pmc.ncbi.nlm.nih.gov/articles/PMC7181455/]
4. Inosine as a Tool to Understand and Treat Central Nervous System Disorders: A Neglected Actor? Francisney Pinto Nascimento, Sérgio José Macedo-Júnior, Fernanda Rocha Lapa-Costa, Fernando Cezar-dos-Santos, Adair R S Santos, 2021. [https://pmc.ncbi.nlm.nih.gov/articles/PMC8421806/]
5. Nitric oxide metabolites and interleukin-6 in cerebrospinal fluid from multiple sclerosis patients. Dj Miljkovic, J Drulovic, V Trajkovic, S Mesaros, I Dujmovic, et al, 2002. [https://pubmed.ncbi.nlm.nih.gov/12099927/]
6. Growing Evidence of a Proven Mechanism Shows Vitamin K2 Can Impact Health Conditions Beyond Bone and Cardiovascular, Katarzyna Maresz, 2021. [https://pmc.ncbi.nlm.nih.gov/articles/PMC8483258/]
7. Associations of cholecystectomy with metabolic health changes and incident cardiovascular disease: a retrospective cohort study. Sangwoo Park, Seogsong Jeong, Sun Jae Park, Jihun Song, Sung Min Kim, et al, 2024. [https://www.nature.com/articles/s41598-024-53161-6]
9. Revisiting the interconnection between lipids and vitamin K metabolism: insights from recent research and potential therapeutic implications: a review. Jing Tan, Ying Li, 2024. [https://pmc.ncbi.nlm.nih.gov/articles/PMC10763176/]
10. Tamsulosin-induced Hyperprolactinemia in a Patient with Multiple Sclerosis: A Case Report.
Ahmet Görgel, Ahmet Soylu, Sacit Görgel, Mehmet Tecellioğlu, Mehmet Sarıer, 2019. [https://jurolsurgery.org/articles/tamsulosin-induced-hyperprolactinemia-in-a-patient-with-multiple-sclerosis-a-case-report/jus.galenos.2018.2436]
11. Dopaminergic Receptor Targeting in Multiple Sclerosis: Is There Therapeutic Potential? Mikhail Melnikov, Mikhail Pashenkov, Alexey Boyko, 2021. [https://pmc.ncbi.nlm.nih.gov/articles/PMC8157879/]
12. Hyperprolactinemia, Cleveland Clinic, 2024. [https://my.clevelandclinic.org/health/diseases/22284-hyperprolactinemia]]
13. Vitamin K2-MK-7 improves nitric oxide-dependent endothelial function in ApoE/LDLR−/− mice. Anna Bar, Kamil Kus, Angelika Manterys, Bartosz Proniewski, Magdalena Sternak, et al, 2019. [https://www.sciencedirect.com/science/article/abs/pii/S153718911930031X]
14. Nitric oxide-matrix metaloproteinase-9 interactions: Biological and pharmacological significance: NO and MMP-9 interactions. Shane O’Sullivan, Carlos Medina a, Mark Ledwidge b, Marek W. Radomski a, John F. Gilmer, et al, 2014. [https://www.sciencedirect.com/science/article/pii/S0167488913004254]
15. Vitamin K2 Suppresses Proliferation and Inflammatory Cytokine Production in Mitogen-Activated Lymphocytes of Atopic Dermatitis Patients through the Inhibition of Mitogen-Activated Protein Kinases. Meiyu Zhang, Taro Miura, Shunsuke Suzuki, Masako Chiyotanda, Sachiko Tanaka, et al, 2021. [https://pubmed.ncbi.nlm.nih.gov/33390552/]
16. Effects of Vitamin D and K on Interleukin-6 in COVID-19. Margot P. J. Visser, Anton S. M. Dofferhoff, Jody M. W. van den Ouweland, Henny van Daal, Cornelis Kramers, et al, 2022. [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2021.761191/full]
17. The biological responses of vitamin K2: A comprehensive review. Quanxiang Yan, Tao Zhang, Christine O’Connor, James W Barlow, John Walsh, et al, 2023. [https://pmc.ncbi.nlm.nih.gov/articles/PMC10084986/]
18. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Griselda A Cabral-Pacheco 1, Idalia Garza-Veloz 1,*, Claudia Castruita-De la Rosa 1, Jesús M Ramirez-Acuña 1, Braulio A Perez-Romero, et al, 2020. [https://pmc.ncbi.nlm.nih.gov/articles/PMC7767220/]
19. Zinc-Binding Cysteines: Diverse Functions and Structural Motifs. Nicholas J Pace 1, Eranthie Weerapana, 2014. [https://pmc.ncbi.nlm.nih.gov/articles/PMC4101490/]
20. Cysteine-Zn2+ complexes: unique molecular switches for inducible nitric oxide synthase-derived NO. KLAUS-D. KRÖNCKE, 2001. [https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fj.01-0240hyp]
21. Nitric Oxide Regulation of MMP-9 Activation and Its Relationship to Modifications of the Cysteine Switch. Sean M McCarthy, Peter F Bove, Dwight E Matthews, Takaaki Akaike, Albert van der Vliet, 2011. [https://pmc.ncbi.nlm.nih.gov/articles/PMC3030771/]
22. Biological role of matrix metalloproteinases: a critical balance. S. Löffek O. Schilling C-W. Franzke ,2011. [https://publications.ersnet.org/content/erj/38/1/191]
23. Neurovascular Matrix Metalloproteinases and the Blood-Brain Barrier. Ji Hae Seo, Shuzhen Guo, Josephine Lok, Deepti Navaratna, Michael J Whalen, Kyu-Won Kim, Eng H Lo, 2012. [https://pmc.ncbi.nlm.nih.gov/articles/PMC3814178/]
24. The Significance of Matrix Metalloproteinases in the Immunopathogenesis and Treatment of Multiple Sclerosis, Abbas Mirshafiey, Babak Asghari, Ghasem Ghalamfarsa, Farhad Jadidi-Niaragh, Gholamreza Azizi, 2014. [https://pmc.ncbi.nlm.nih.gov/articles/PMC3916267/]
25. Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity. Ha Vy Thi Vo, Yen Thi Nguyen, Namdoo Kim, Hyuck Jin Lee, 2023. [https://pmc.ncbi.nlm.nih.gov/articles/PMC10707015/]
26. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Martina B Sintzel, Mark Rametta, Anthony T Reder, 2017. [https://pmc.ncbi.nlm.nih.gov/articles/PMC5990512/]
27. Growing Evidence of a Proven Mechanism Shows Vitamin K2 Can Impact Health Conditions Beyond Bone and Cardiovascular. Katarzyna Maresz, 2021. [https://pmc.ncbi.nlm.nih.gov/articles/PMC8483258/]
28. The Biosynthesis of Menaquinone-4: How a Historic Biochemical Pathway Is Changing Our Understanding of Vitamin K Nutrition. Martin J Shearer, 2022. [https://www.researchgate.net/publication/363196923_The_Biosynthesis_of_Menaquinone-4_How_a_Historic_Biochemical_Pathway_Is_Changing_Our_Understanding_of_Vitamin_K_Nutrition]
29. Study Finds Vitamin K2 Repairs Nerve Cells By Repairing Mitochondrial Dysfunction, magazine article, 2022. [https://www.prohealth.com/blogs/breaking-news/study-finds-vitamin-k2-repairs-nerve-cells-by-repairing-mitochondrial-dysfunction]
30. Neuroprotective effect of Vitamin K2 against gut dysbiosis associated cognitive decline. Kaberi Chatterjee,Papiya Mitra Mazumder a,Suparna Roy Sarkar,Rajdeep Saha, Amrita Chatterjee, Biswatrish Sarkar, Sugato Banerjee, 2023. [https://www.sciencedirect.com/science/article/abs/pii/S0031938423001774]
31. The biological responses of vitamin K2: A comprehensive review, Quanxiang Yan, Tao Zhang, Christine O’Connor, James W Barlow, John Walsh, et al, 2023. [https://pmc.ncbi.nlm.nih.gov/articles/PMC10084986/]
33. Amyloidogenic and Neuroinflammatory Molecular Pathways Are Contrasted Using Menaquinone 4 (MK4) and Reduced Menaquinone 7 (MK7R) in Association with Increased DNA Methylation in SK-N-BE Neuroblastoma Cell Line. Michela Orticello, Rosaria A Cavallaro, Daniele Antinori, Tiziana Raia, Marco Lucarelli, Andrea Fuso, 2023. [https://pubmed.ncbi.nlm.nih.gov/38201262/]
34. Vitamin K2 in Health and Disease: A Clinical Perspective. Tao Zhang, Christine O’Connor, Helen Sheridan, James W Barlow , 2024. [https://pmc.ncbi.nlm.nih.gov/articles/PMC11172246/]
35. Scientists Identify Specific Bacteria Linked to Multiple Sclerosis, David Nield, 2025. [https://www.sciencealert.com/scientists-identify-specific-bacteria-linked-to-multiple-sclerosis]
36. Multiple sclerosis and gut microbiota: Lachnospiraceae from the ileum of MS twins trigger MS-like disease in germ free transgenic mice-An unbiased functional study. Hongsup Yoon, Lisa Ann Gerdes , Florian Beigel, Yihui Sun,Janine Kövilein, et al, 2025. [https://pubmed.ncbi.nlm.nih.gov/40258140/]
37. Inactive Matrix Gla-Protein Is Associated With Arterial Stiffness in an Adult Population–Based Study. Edward Pivin, Belen Ponte, Menno Pruijm, Daniel Ackermann, Idris Guessous, et al, 2015. [https://www.ahajournals.org/doi/10.1161/hypertensionaha.115.05177]
38. Induction of epstein-barr virus (EBV) lytic cycle in vitro causes lipid peroxidation, protein oxidation and DNA damage in lymphoblastoid B cell lines. Bochra Gargouri, Rihab Nasr, Malek Mseddi, Riadh benmansour, saloua Lassoued, 2011. [https://link.springer.com/article/10.1186/1476-511X-10-111]
40. Peroxidation of lipoproteins in multiple sclerosis. Gianna Ferretti, Tiziana Bacchetti, 2011. [https://pubmed.ncbi.nlm.nih.gov/21967834/]
41. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Jillian P Rhoads, Amy S Major, 2019. [https://pmc.ncbi.nlm.nih.gov/articles/PMC6527110/]
42. Hyperphosphatemia, Preeti Rout, Ishwarlal Jialal. 2023. [https://www.ncbi.nlm.nih.gov/books/NBK551586/]
43. Vitamin K2 In Health and Disease: A Clinical Perspective. Tao Zhang, Christine O’Connor, Helen Sheridan, James W Barlow, 2024. [https://pmc.ncbi.nlm.nih.gov/articles/PMC11172246/]
44. Phosphate Binders affect Vitamin K concentration by undesired binding, an in vitro study. A Neradova, S P Schumacher, I Hubeek, P Lux, L J Schurgers, M G Vervloet, 2017. [https://pubmed.ncbi.nlm.nih.gov/28464802/]
45. Combining phosphate binder therapy with vitamin K2 inhibits vascular calcification in an experimental animal model of kidney failure. Aegida Neradova, Grzegorz Wasilewski, Selene Prisco, Peter Leenders, Marjolein Caron,et al, 2022. [https://pubmed.ncbi.nlm.nih.gov/34718756/]
46. The link between statins, atherosclerosis, and lack of vitamin K. Article, 2021. [https://www.healthandscience.eu/us/archive-us/article-archive/3005-the-link-between-statins-atherosclerosis-and-lack-of-vitamin-k-us]
47. A combined experimental and theoretical study of radon solubility in fat and water. Elvira P. Sanjon, Andreas Maier, Annika Hinrichs, Gerhard Kraft, Barbara Drossel, Claudia Fournier, 2019. [https://www.researchgate.net/publication/334647108_A_combined_experimental_and_theoretical_study_of_radon_solubility_in_fat_and_water]
48. Gut-Brain Connection: Eisenbergiella tayi and Lachnoclostridium Intestinal Bacteria Linked to Multiple Sclerosis. Article medically reviewed By Vikas Londhe M.Pharm (Pharmacology), 2025. [https://pharmacally.com/gut-brain-connection-eisenbergiella-tayi-and-lachnoclostridium-intestinal-bacteria-linked-to-multiple-sclerosis/]
49. Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. Liang Wei Wang, Zhonghao Wang, Ina Ersing, Luis Nobre, Rui Guo, Sizun Jiang, et al, 2019. [https://pmc.ncbi.nlm.nih.gov/articles/PMC6760809/]
50. The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission, Christopher P Ford, 2015. [https://pmc.ncbi.nlm.nih.gov/articles/PMC4108583/]
51. Processes of Sterile Inflammation, Hua Shen, Daniel Kreisel, Daniel Robert Goldstein, 2013. [https://academic.oup.com/jimmunol/article-abstract/191/6/2857/7966451?redirectedFrom=fulltext]
52. Determination of vitamins K1, MK‐4, and MK‐7 in human serum of postmenopausal women by HPLC with fluorescence detection, Eva Klapkova , Jana Cepova , Katerina Dunovska , Richard Prusa, 2018, [https://pmc.ncbi.nlm.nih.gov/articles/PMC6816853/]
53. Amyloid Proteins and Their Role in Multiple Sclerosis. Considerations in the Use of Amyloid-PET Imaging. Jordi A Matías-Guiu, Celia Oreja-Guevara, María Nieves Cabrera-Martín, Teresa Moreno-Ramos, José Luis Carreras, Jorge Matías-Guiu, 2016. [https://pmc.ncbi.nlm.nih.gov/articles/PMC4814935/]
54. FDA Clears First Blood Test Used in Diagnosing Alzheimer’s Disease. FDA Press Release, 2025. [https://www.fda.gov/news-events/press-announcements/fda-clears-first-blood-test-used-diagnosing-alzheimers-disease]
55. Reduced Vitamin K Status as a Potentially Modifiable Risk Factor of Severe Coronavirus Disease 2019, Anton S M Dofferhoff, Ianthe Piscaer, Leon J Schurgers, Margot P J Visser, Jody M W van den Ouweland, et al, published 2020. [https://academic.oup.com/cid/article/73/11/e4039/5898121?login=false]
56. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Allan Linneberg, Freja Bach Kampmann, Simone Bastrup Israelsen, Liv Rabøl Andersen, Henrik Løvendahl Jørgensen, et al, 2021. [https://pubmed.ncbi.nlm.nih.gov/34207745/]
57. Dramatic Decrease of Vitamin K2 Subtype Menaquinone-7 in COVID-19 Patients. Harald Mangge, Florian Prueller, Christine Dawczynski, Pero Curcic, Zdenka Sloup, et al, 2022. [https://pmc.ncbi.nlm.nih.gov/articles/PMC9312339/]
58. Higher levels of circulating desphospho-uncarboxylated matrix Gla protein over time are associated with worse survival: the prospective Maastricht Intensive Care COVID cohort. Mark M G Mulder, Joep Schellens, Jan-Willem E M Sels, Frank van Rosmalen, Anne-Marije Hulshof, et al, 2023. [https://pmc.ncbi.nlm.nih.gov/articles/PMC10726599/]
59. The Association Between Alpha-1 Adrenergic Receptor Antagonists and In-Hospital Mortality from COVID-19. Liam Rose, Laura Graham, Allison Koenecke, Michael Powell, Ruoxuan Xiong, et al, 2021. [https://pmc.ncbi.nlm.nih.gov/articles/PMC7781337/]
60. Influence of vitamin K2 on lipid precursors of inflammation and fatty acids pathway activities in HepG2 cells. Adrian Kołakowski, Piotr Franciszek Kurzyna, Wiktor Bzdęga, Hubert Żywno, Ewa Harasim-Symbor, et al, 2021. [https://pubmed.ncbi.nlm.nih.gov/34837768/]